3 Why are gas bubbles observed when sodium bicarbonate is added to the mixture. Pure isoamyl acetate or mixtures of isoamyl.

Pavia Experiment 14a Starting Materials Solubility Ionization Ld 50 Product Properties Solubility Boiling Point Ionization Reactions Overall Ppt Download
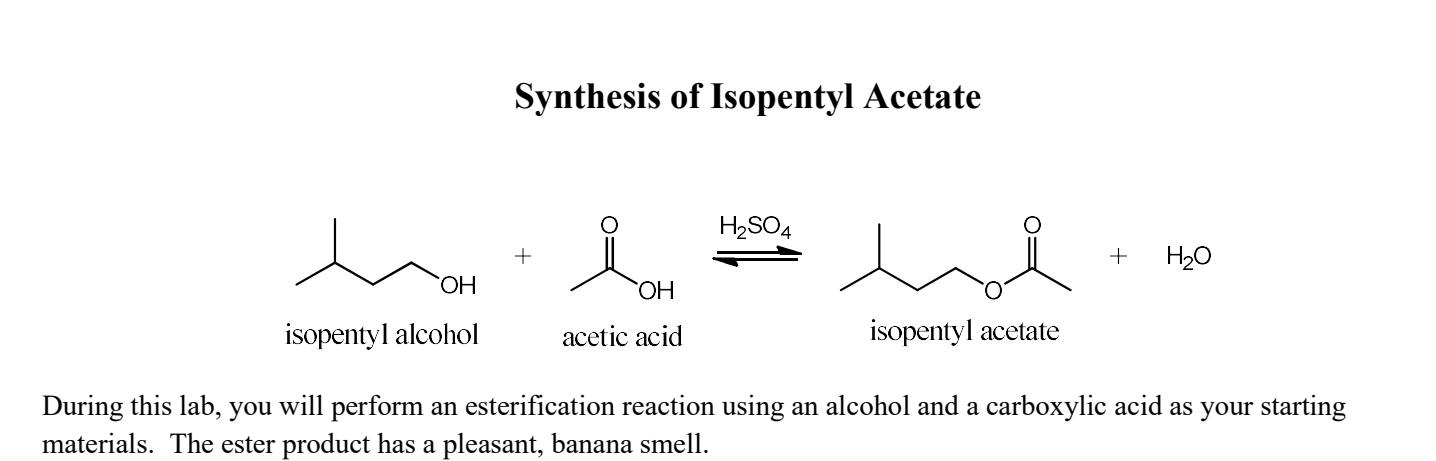
The esterification of isopentyl acetate from isopentyl alcohol and acetic acid.

. What happens is the alcohol can act as the nucleophile in this favorable. Mass of Iso pentyl alcohol mass of vial cap aaisopentyl alcohol - mass of vial cap Mass of Isopentyl alcohol 26 - 22. Protonation of the carbonyl oxygen addition of the alcohol to create a tetrahedral intermediat e.
In the flask place 30 mL of isopentyl alcohol AKA. Produces a banana aroma. Write a balanced chemical equation that shows how each acid was neutralized in this experiment.
If so how do we find the mass of acetic acid in this experiment volume of acetic. The balanced reaction is shown below. C7H14O2 a fragrant substance with the odor of bananas.
The percent yield the product isopentyl acetate was 41 percent with a 97 percent purity and 40 corrected yield. Swirl the flask and carefully add 4 mL of conc. If the yield from the reaction of acetic acid with isopentyl alcohol is 45percent how many grams of isopentyl acetate are formed from 368 g of acetic acid and 471 g of isopentyl alcohol.
Write the overall balanced equation and mechanism for the synthesis of isopentyl acetate from isopentyl alcohol and acetic acid. The reaction with 8 mL of acetic acid with 5 g of isopentyl alcohol underwent fischer esterification using sulfuric acid as a catalyst. The mechanism goes as follows the unsaid step being to protonate the carbonyl of course because of the high pi electron density.
Mass of Isopentyl Alcohol calculation. Isoamyl acetate also known as isopentyl acetate is an organic compound that is the ester formed from isoamyl alcohol and acetic acidIt is a colorless liquid that is only slightly soluble in water but very soluble in most organic solvents. It derives from an isoamylol.
Acetic acid CH3COOH reacts with isopentyl alcohol C5H12O to yield the ester isopentyl acetate which has the odour of bananas. CH3COOH C5H12O C7H14O2 H2O. 437 g X 1 mol 8815 gmol 00459 mol Appendix B.
Calculating Theoretical Yield of Isopentyl Acetate Moles of limiting reagent X molar ratio X molecular weight of product 1 mol theoretical yield 00459 X 13019 1 mol 644 g. Equation - A C CH O Chlook isopentyl H2sox C j Hly 2 Acetic ISopenty. CH 3 COOHCH 3 CH 2 OHCH 3 COOC 2 H 5 H 2 O.
If the yield from the reaction is 45 how many grams of isopentyl acetate are formed when 377 g of. Acetic acid CH3CO2H C5H12O rightarrow C7H14O2 H2OExpress your answer in two significant figures. Isopentyl alcohol 3-methyl-1-butanol and acetic acid ethanoic acid see Figure 3.
Include all reagents and products but not solvents. The OH is lost from the acetic acid and one H is lost from the alcohol. H 2 SO 4 CAUTION.
Isoamyl acetate has a strong odor which is described as similar to both banana and pear. O H 3-meth ylbutan-1-ol O O H acetic acid O O isop en tl ac H 2 O Procedure. Kinetic modeling was also done using First Principle model and found that the kinetic constant for k 1 and k 2 value equals to.
H 2SO 4 9808 5 mL 1841 5 NaHCO 3 8401 250 mL 10018 sat. Glacial Acetic Acid. Alcohol Acid Acetate H OH CHROM HSO4 Iso pentyl Iso pentyl Acetate.
NaCl 10 mL Na 2SO 4 anhydr 14204. Experts are tested by Chegg as specialists in their subject area. Now take a look at the balanced chemical equation.
4 If you started with 80 mL of isopentyl alcohol and 80 mL glacial acetic acid in this reaction which substance would be your limiting reactant. Isoamyl alcohol and 3-methyl-1-butanol 40 mL of glacial acetic acid and 08 mL of concentrated sulfuric acid. It has a role as a metabolite and a Saccharomyces cerevisiae metabolite.
I between acetic anhydride and isoamyl alcohol and ii between acetic acid and isoamyl alcohol. CH3COOHl C5H11OHl CH3COOC5H11 l H2Ol Acetic acid CH3COOH and isoamyl alcohol C5H1OH will react in a 11 mole ratio to produce isoamyl acetate CH3COOC5H11 and water. Balanced equation for reaction of acetic acid with isopentyl alcohol.
Isoamyl acetate is a natural product found in Vitis rotundifolia Nicotiana bonariensis and other organisms with data available. Acetic acid anhydr 6005 25 mL 118 1049 isopentyl acetate 13019 product 142 0876 isopentyl alcohol 8815 20 mL 130 0809 conc. If a student reacts 550 mL of acetic acid with 235 mL of isopentyl alcohol and obtains 325 mL of isopentyl acetate as the product what was the percent yield of the reaction.
Reactions took place which are. We review their content and use your feedback to keep the quality high. This reaction yielded 3 g of isopentyl acetate also known as banana oil and water as a by product.
H 3C O O H HOCH 2 CHCH H 3C O O CH 2 2CHCH 3 2 HO H Acetic acid ethanoic acid MW 601 d 105 bp 118 o C Isopentyl alcohol 3-methyl-1-butanol MW 881 d 081 bp 129 oC Isopentyl acetate 3-methyl-1-butyl acetate MW 1302 d 0867 bp 142 o H 2SO 4 Figure 3. Who are the experts. Essentially what you have is an acid-catalyzed dehydration.
85 mL X 1 g1 mL X 1 mol 6005 gmol 0142 mol Isopentyl Alcohol. Place 15 mL 122 g O138 mole of isopentyl alcohol in a 1OO-mL round-bottom flask and add 2O mL 21 g 035 mole of glacial acetic acid. Synthesis OF Isopentyl Acetate from isopentyl Alcohol Acetic Acid in presence OF Acid the Preocess is known as Estenification.
Include name strutural and condensed formuala and molar mass. Assemble a reflux apparatus using a 50 or a 100 mL round-bottom flask and an air condenser. If the yield from the reaction is 45 how many grams of isopentyl acetate are formed when 377 g of acetic acid and 746 grams of isopentyl alcohol are reacted.
Alcohol Mechanism -. Acetic acid CH3COOH reacts with isopentyl alcohol C5H12O to yield the ester isopentyl acetate which has the odour of bananas. H H20 HO HO acetic acid isopentyl alcohol isopentyl acetate bp 142 C bp 118 C bp 129 C MW 600 MW 882 MW 1302 d.
Isoamyl acetate is the acetate ester of isoamylol. Some of key features of the esterification mechanism are the the. Simply put theoretical yield is what you get when the reaction has a 100 yield.

Oneclass Isopentyl Acetate Can Be Prepared By An Esterification Reaction Using Acetic Acid And Isope
Solved Synthesis Of Isopentyl Acetate H2so4 H20 On Oh Chegg Com

0 Comments